Video Tutorial Amines as Bases
Quick Notes Amines
- Amines contain a carbon chain bonded to a nitrogen atom that is bonded to either hydrogen or another carbon chain (alkyl).
- Amines can be primary, secondary or tertiary (quarternary amines exist as positively charged ions).
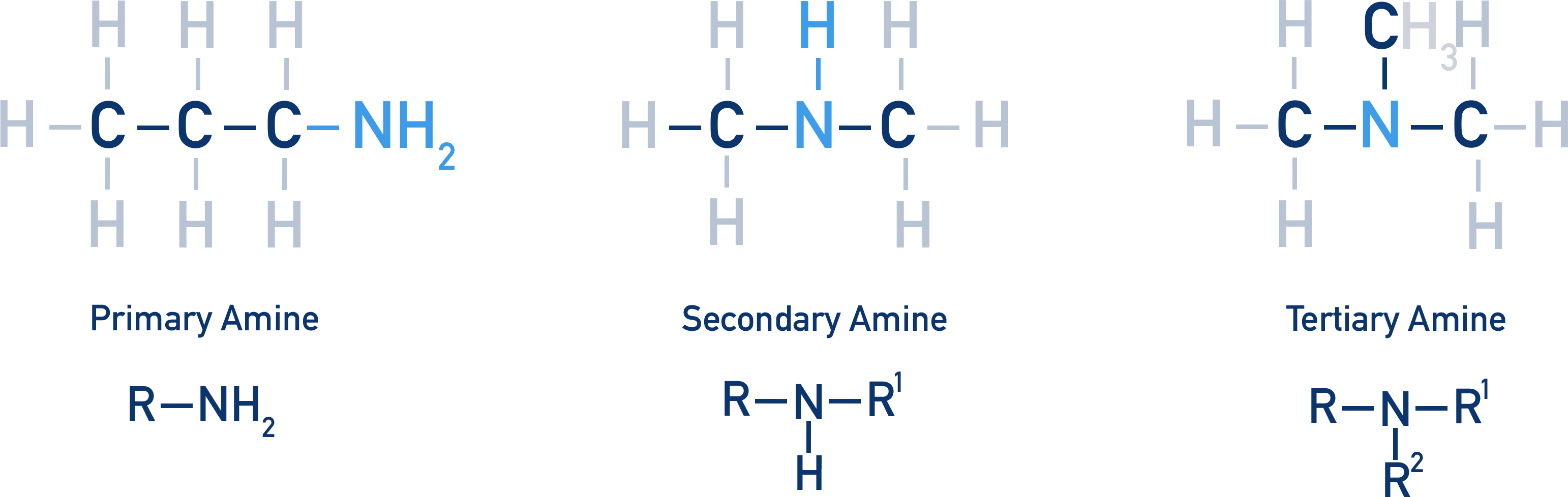
- A lone pair of electrons on the nitrogen atom enables amines to act as bases (and nucleophiles).
- Primary amines are less basic than secondary amines, and secondary amines are less basic than teritiary amines.
- This is due to the positive inductive effect of alkyl chains.
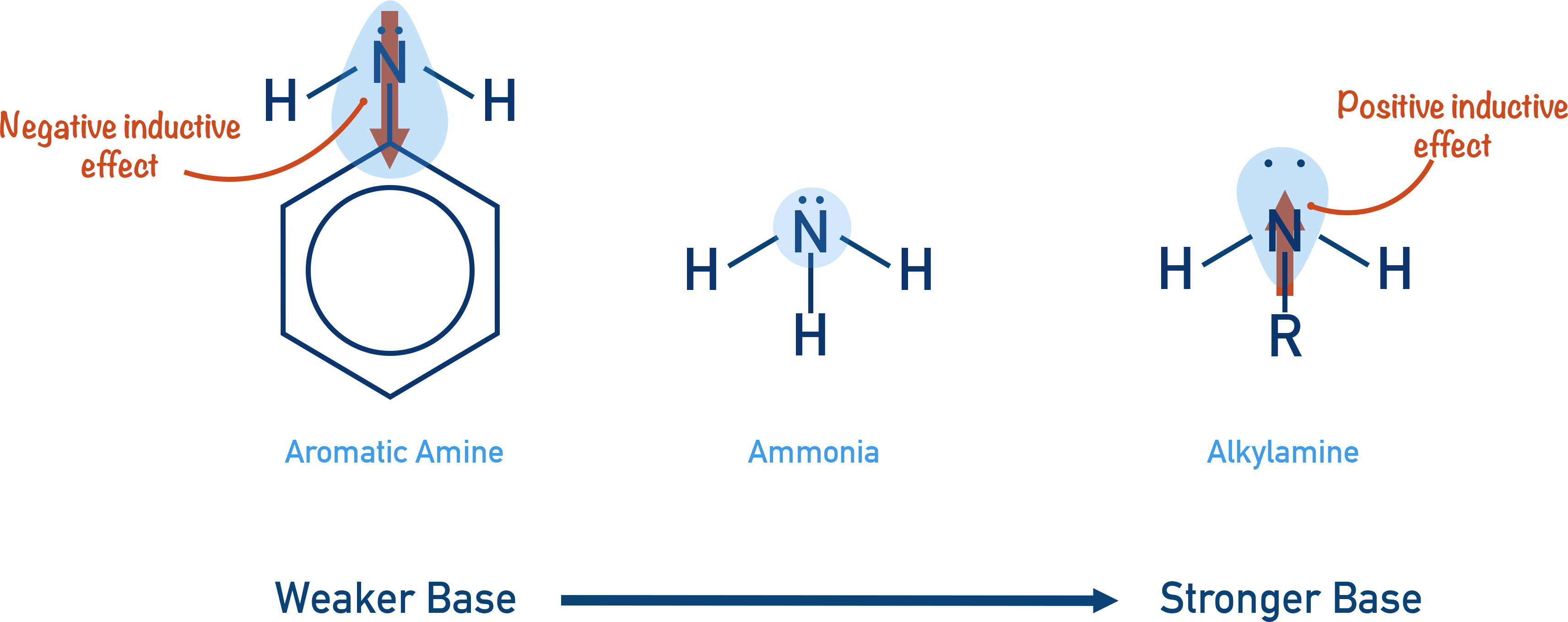
- Aromatic amines are weak bases as the benzene ring is electron withdrawing, making the lone pair of electrons on the nitrogen less available to accept a proton.
- Primary amines are less basic than secondary amines, and secondary amines are less basic than teritiary amines.
Full Notes Amines
Amine functional groups derive from ammonia (NH3). They are useful in organic reactions, as they contain a nitrogen atom (with a lone pair of electrons) that can act as a nucleophile. This means compounds with an amine group can act as nucleophiles. The lone pair of electrons on the nitrogen can also accept protons, making them basic.
Primary, Secondary and Tertiary Amines
Amines can be primary, secondary or tertiary.
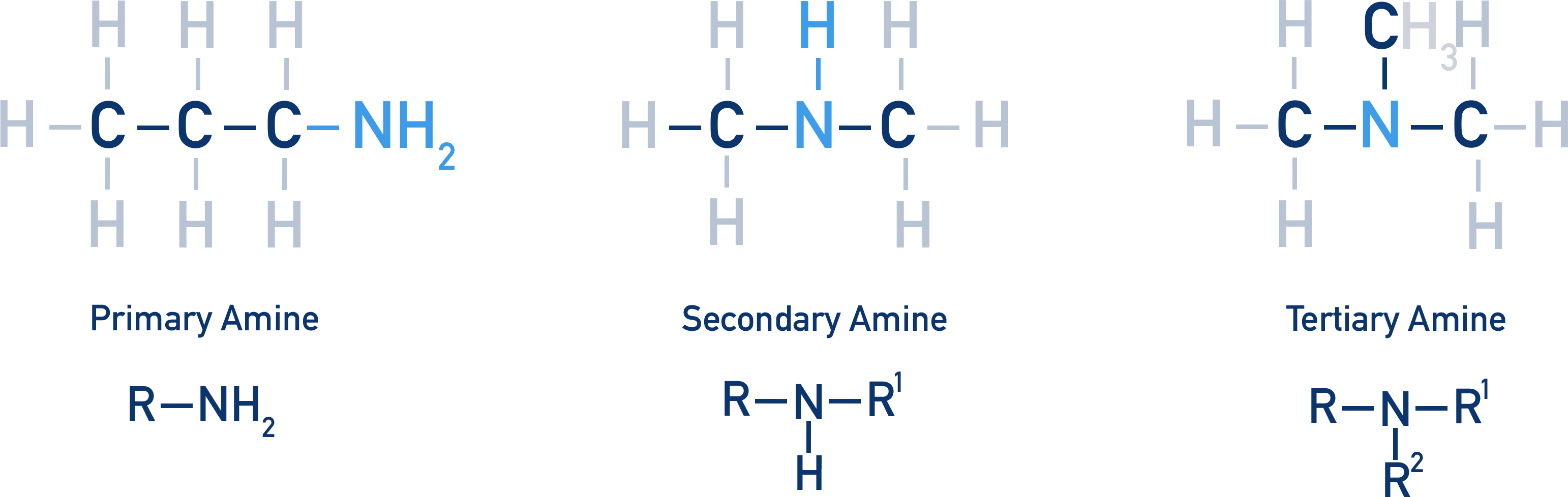
A primary amine has an NH2 group at the end of a carbon chain; the nitrogen is bonded to one carbon atom.
A secondary amine has an NH group; the nitrogen is bonded to two carbon atoms.
A tertiary amine has no hydrogens bonded to the nitrogen; the nitrogen is bonded to three carbon atoms.
Basicity of Amines
How basic an amine is (basicity, see acids and bases) can be predicted from its structure.
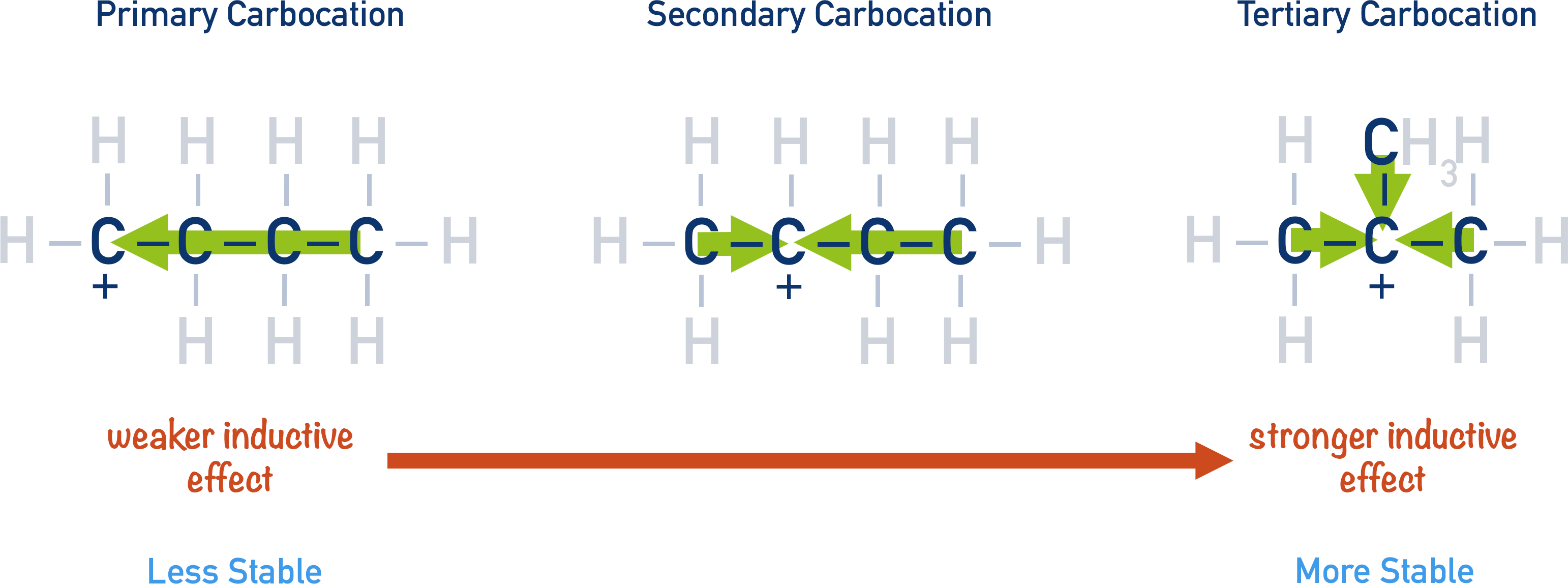
Alkyl groups are slightly electron donating. This is why primary carbocations are less stable than secondary carbocations. Tertiary carbocations are the most stable as the positive charge is ‘stabilised’ by the slight donation of electrons from all three of the alkyl groups.
Bases accept protons because they have a lone pair of electrons. The more ‘available’ the lone pair is, the easier it is for a proton to be accepted, making the molecule more basic.
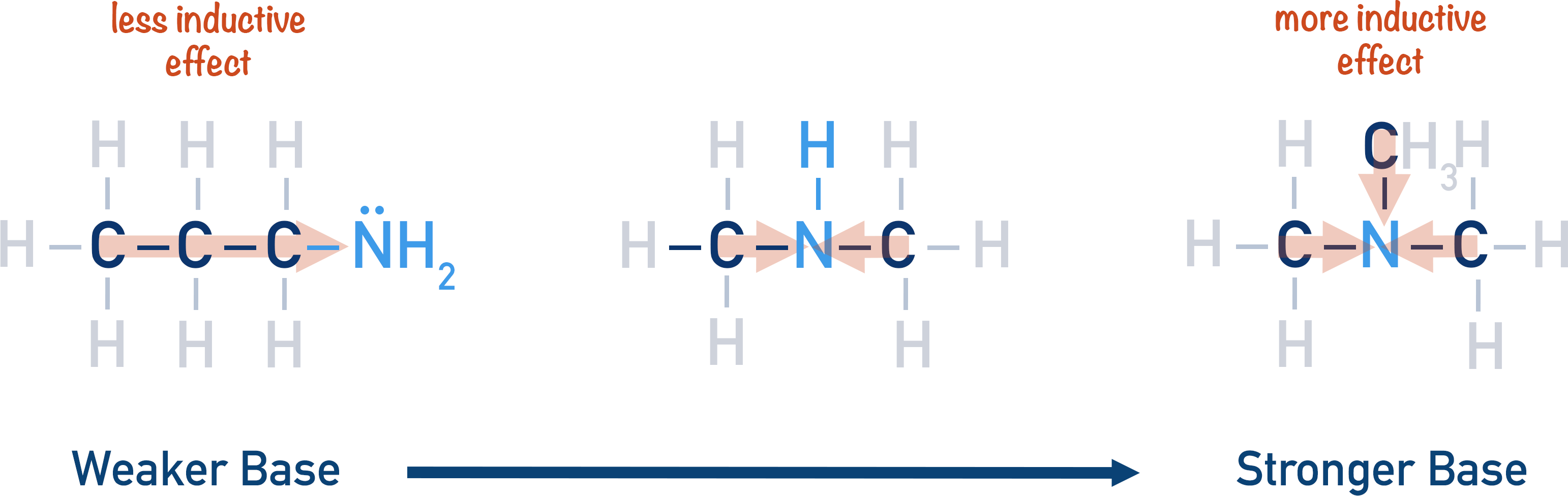
The slight electron donating effect of alkyl groups increases the basicity of an amine, as it makes the lone pair on the nitrogen atom more available and likely to accept a proton (act as a base).
The more alkyl groups ‘pushing’ electron density towards the nitrogen atom (positive inductive effect), the easier it is for the lone pair of electrons to accept a proton. Primary amines only have one alkyl group, so they have less of the pushing effect than secondary amines. Tertiary amines have three alkyl groups pushing electron density towards the nitrogen atom, which makes tertiary amines the most basic.
An aromatic amine (an amine group on a benzene ring) is less basic than an aliphatic (non-aromatic) amine because the benzene ring has an electron withdrawing effect and pulls electron density away from the nitrogen atom. The lone pair is less available for protons to bond to, so the amine is less basic.