General Properties of Transition Metals
Quick Notes
- Transition metals have an incomplete d sub-shell in their atoms or ions (first row Ti–Cu).
- Key properties of transition metals include:
- Complex formation – transition metals form complexes with ligands.
- Formation of coloured ions – due to d-orbital electron transitions.
- Variable oxidation states – they can form multiple oxidation states.
- Catalytic activity – can often act as catalysts.
- Ligands are molecules or ions that donate a pair of electrons to a metal ion to form a co-ordinate (dative covalent) bond.
- A complex consists of a central metal atom or ion surrounded by ligands.
- Co-ordination number refers to the number of co-ordinate bonds to a central metal atom or ion in a complex.
Full Notes
Transition metals and complex ions have been outlined in more detail
here.
This page is just what you need to know for AQA A-level Chemistry :)
What Are Transition Metals?
Transition metals are elements in the d-block that have an incomplete d sub-shell in their atoms or ions.

For A-Level students, the AQA syllabus focuses on Ti–Cu.
- Titanium (Ti)
- Vanadium (V)
- Chromium (Cr)
- Manganese (Mn)
- Iron (Fe)
- Cobalt (Co)
- Nickel (Ni)
- Copper (Cu)
Key Properties of Transition Metals
Complex Formation
Transition metals form complex ions when surrounded by ligands.
A complex ion consists of a central metal ion bonded to ligands via co-ordinate (dative covalent) bonds.
Formation of Coloured Ions
Transition metal ions are often coloured in solution.
Variable Oxidation States
Transition metals can form multiple oxidation states.
This happens because d-electron orbitals are close in energy, allowing different numbers of electrons to be lost.
| Element | Common oxidation states (examples) |
|---|---|
| Ti | +2, +3, +4 |
| V | +2, +3, +4, +5 |
| Cr | +2, +3, +6 |
| Mn | +2, +4, +7 |
| Fe | +2, +3 |
| Co | +2, +3 |
| Ni | +2, +3 |
| Cu | +1, +2 |
Catalytic Activity
Transition metals and their compounds act as catalysts due to their ability to change oxidation states and adsorb reactants onto their surface.
| Catalyst | Industrial / reaction use |
|---|---|
| Fe (solid iron) | Haber process (N2 + 3H2 ⇌ 2NH3) |
| V2O5 | Contact process (2SO2 + O2 ⇌ 2SO3) |
| Ni (finely divided) | Hydrogenation of alkenes |
| MnO2 | Catalytic decomposition of H2O2 |
Remember – Catalysts lower activation energy and speed up reactions.
Ligands, Complex Ions, and Co-ordination Number
What Is a Ligand?
A ligand is a molecule or ion that donates a pair of electrons to a central metal ion to form a co-ordinate bond.
Example:Water molecules (H2O) are able to act as ligands as the oxygen atom can use one of its lone pairs of electrons to form a co-ordinate bond to a central metal atom or ion.
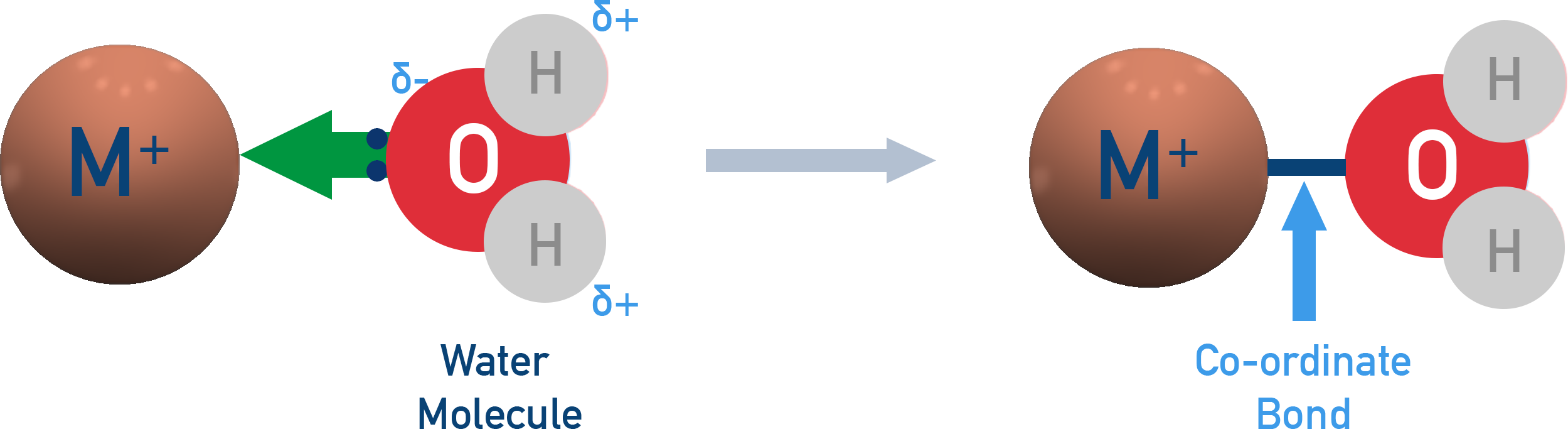
Types of Ligand (see ligand substitution for more detail):
- Monodentate: Donates one pair of electrons (e.g., H2O, NH3, Cl−).
- Bidentate: Donates two pairs of electrons (e.g., ethane-1,2-diamine).
- Multidentate: Donates multiple pairs of electrons (e.g., EDTA4−).
What Is a Complex Ion?
A complex ion consists of a central transition metal ion surrounded by ligands via co-ordinate bonds.
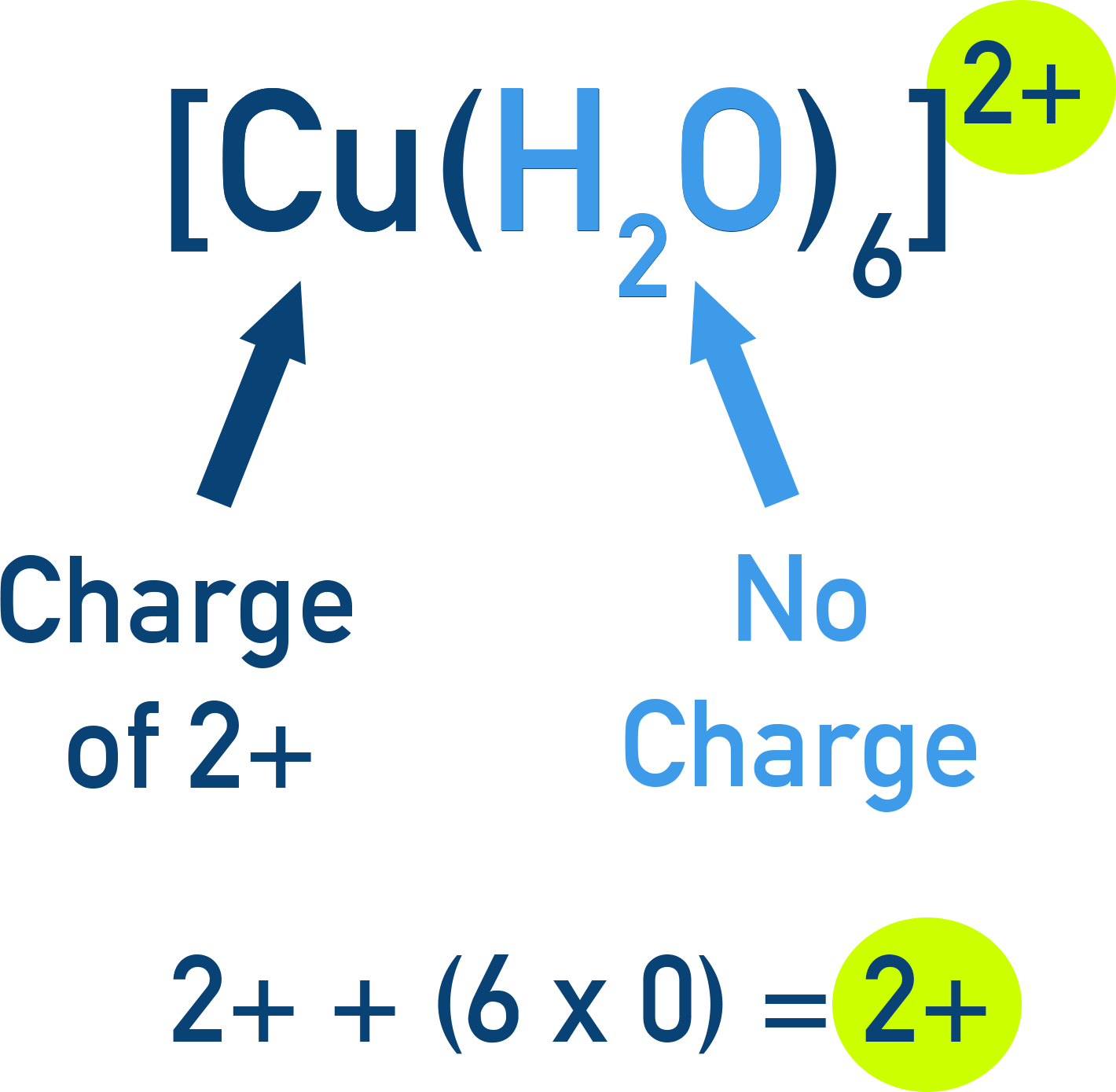
Example Example complex ion: [Cu(H2O)6]2+ (hexaaquacopper(II) ion)
![AQA A-Level Chemistry diagram of the hexaaquacopper(II) complex [Cu(H2O)6]2+](images/coppercomplex.png)
The formulas of complex ions are written in square brackets with the overall charge of the complex ion shown as a superscript.
Co-ordination Number
Co-ordination number refers to the number of co-ordinate bonds around a central metal ion and determines the geometry (shape) of the complex.

| Co-ordination number | Common geometry | Typical example |
|---|---|---|
| 6 | Octahedral | [M(H2O)6]n+ |
| 4 | Tetrahedral | [CuCl4]2− |
| 4 | Square planar | [Pt(NH3)2Cl2] |
| 2 | Linear | [Ag(NH3)2]+ |
Most complexes have a co-ordination number of 6 (octahedral) or 4 (tetrahedral or square planar).

Don’t confuse co-ordination number with the number of ligands in a complex ion! Sometimes the number of ligands can be different to the co-ordination number (for complexes with bidentate and multidentate ligands in).
Summary Table
| Property | What it means |
|---|---|
| Complex formation | Ligands donate lone pairs to a central metal ion, forming co-ordinate bonds |
| Coloured ions | d-electron transitions absorb visible light |
| Variable oxidation states | Similar energy d-orbitals allow different numbers of electrons to be lost |
| Catalysis | Change oxidation state and/or adsorb reactants to lower Ea |
| Co-ordination number | Number of co-ordinate bonds around the metal centre |
