Hess’s Law
Quick Notes
- Hess’s Law states that the total enthalpy change for a reaction is the sum of the enthalpy changes for each step in a reaction pathway.
- The overall enthalpy change is the same regardless of the pathway, provided initial and final conditions are the same.
- It is based on the law of conservation of energy: energy is neither created nor destroyed.
Full Notes
What is Hess’s Law?
Many chemical and physical processes occur in multiple steps, each with its own enthalpy change (ΔH).
Definition:
Hess’s Law states: “The total enthalpy change for a reaction is the same, regardless of the pathway taken, provided the initial and final conditions are the same.”
This means we can calculate the overall enthalpy change by adding the ΔH values of individual steps:
ΔH°Overall = ΔH1 + ΔH2 + ΔH3 + …
Why It Works
Hess’s Law is a consequence of the first law of thermodynamics: energy cannot be created or destroyed.
The total energy change (ΔH) depends only on the initial and final states — not on how the reaction gets there. Whether a process happens in one step or several, the total ΔH will be the same if start and end points are the same.

Hess’s Law only applies when the starting and ending conditions — like temperature and pressure — are the same in each path. Always double-check this before applying the law.
How to Use Hess’s Law – Key Rules
-
Reversing a reaction → reverse the sign of ΔH
If a reaction is endothermic in one direction, it becomes exothermic in the reverse.
Example:
If A → B ΔH = +50 kJ
then B → A ΔH = −50 kJ -
Multiplying a reaction → multiply ΔH by the same factor
Enthalpy is an extensive property, so it scales with the amount of substance.
Example:
If A → B ΔH = +50 kJ
then 2A → 2B ΔH = +100 kJ -
Adding reactions → add their ΔH values
The overall enthalpy change is the sum of steps.
Example:
If A → B ΔH = +50 kJ
and B → C ΔH = −20 kJ
then A → C ΔH = +30 kJ
Worked Example: Using Hess’s Law
Question: Use the following thermochemical equations to calculate ΔH for the formation of CH4(g) from C(s) and H2(g):
Target equation:
C(s) + 2 H2(g) → CH4(g)
Given equations:
- C(s) + O2(g) → CO2(g) ΔH = −393.5 kJ
- H2(g) + ½O2(g) → H2O(l) ΔH = −285.8 kJ
- CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l) ΔH = −890.3 kJ
- Step 1: Target → Formation of CH4(g)
- Step 2: Rearrange
Reverse equation 3: CO2(g) + 2 H2O(l) → CH4(g) + 2 O2(g) ΔH = +890.3 kJ
Keep equation 1 as is: C(s) + O2(g) → CO2(g) ΔH = −393.5 kJ
Multiply equation 2 by 2: 2 H2(g) + O2(g) → 2 H2O(l) ΔH = −571.6 kJ - Step 3: Add & Simplify
After canceling CO2, H2O, and O2, we get:
C(s) + 2 H2(g) → CH4(g) - Step 4: Add ΔH values
ΔH = +890.3 − 393.5 − 571.6 = −74.8 kJ
Final Answer: ΔH = −74.8 kJ

Be methodical and don't rush! Always check that your final equation matches the target before summing ΔH values. Keep workings very clear!
Other Uses of Hess’s Law
Hess’s Law is useful when direct measurement is difficult – we can use enthalpy cycles to calculate ΔH indirectly.
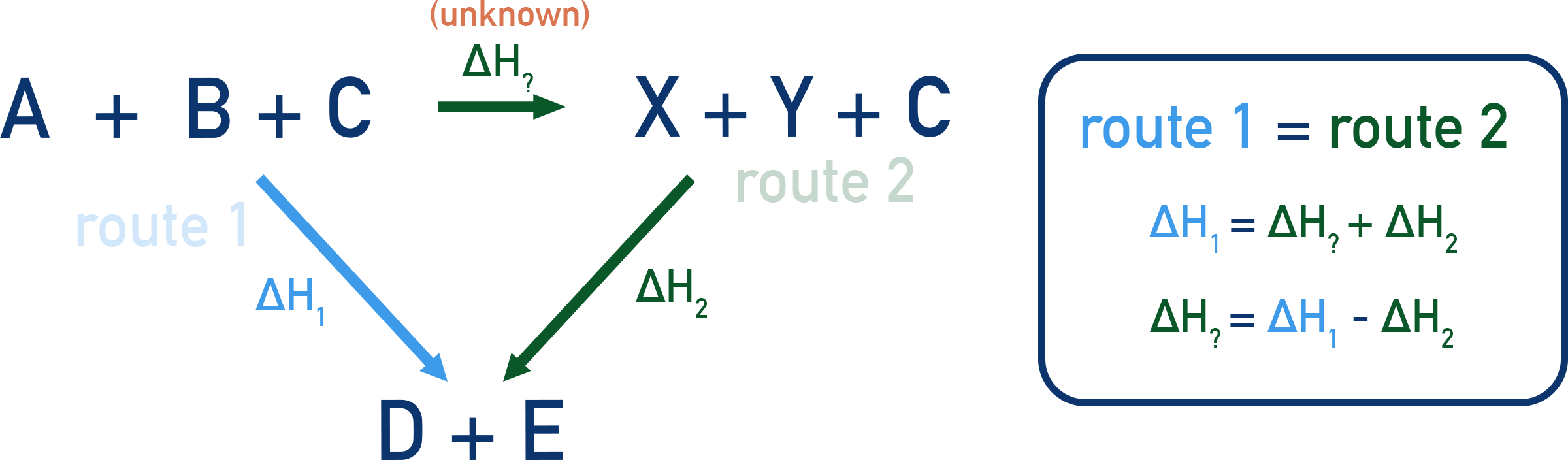
- Enthalpy of formation (ΔHf) calculations
- Enthalpy of combustion (ΔHc) calculations
2. Hess’s Law for Enthalpy of Formation
See Topic 6.8 for more detail.
ΔHf = enthalpy change when one mole of a compound is formed from its elements in standard states.
For elements in standard states, ΔHf = 0.
Formula: ΔHr = ΣΔHf(products) − ΣΔHf(reactants)
Example: Formation of CO2
- C(s) + O2(g) → CO2(g)
- ΔHf(CO2) = −393 kJ mol⁻¹
- ΔHf(O2) = 0
ΔHr = −393 − (0 + 0) = −393 kJ mol⁻¹
3. Hess’s Law for Enthalpy of Combustion
ΔHc = enthalpy change when one mole of a substance is completely burned in oxygen.
Example: Using enthalpies of combustion to find ΔHf of propane (C3H8)
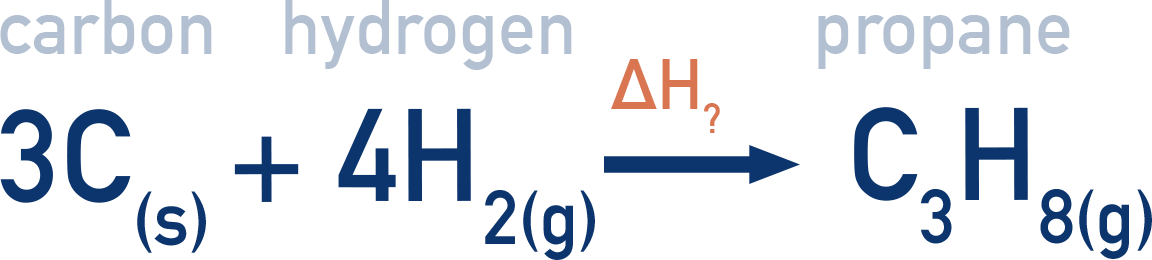
Data:
ΔHc(C) = −394 kJ mol⁻¹
ΔHc(H2) = −286 kJ mol⁻¹
ΔHc(C3H8) = −2220 kJ mol⁻¹
We can combust 3C(s) + 4H2(g) to form 3CO2(g) + 4H2O(l), or combust C3H8(g) to form the same products.
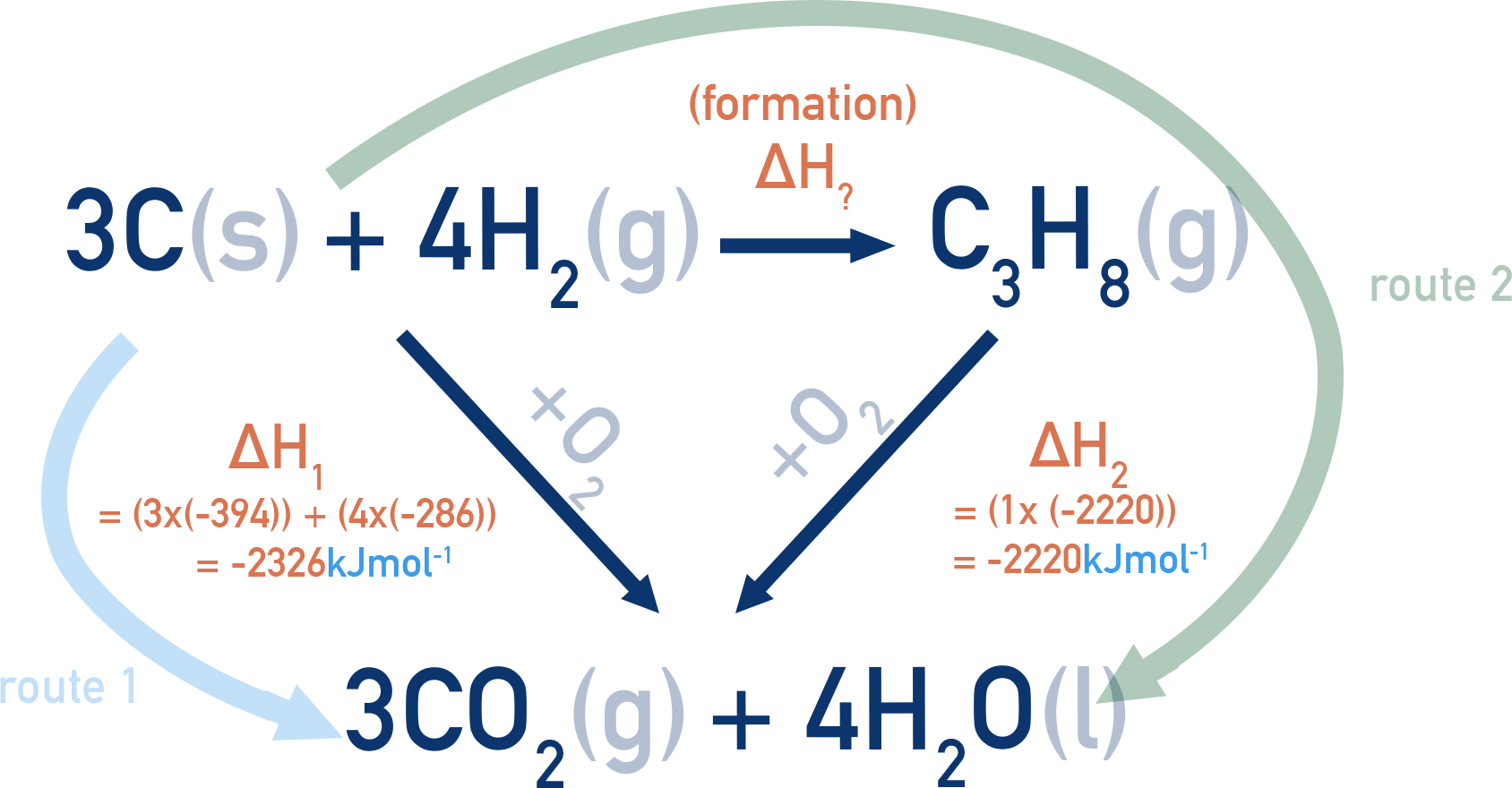
By Hess’s Law: route 1 = route 2
ΔH1 = ΔH? + ΔH2
ΔH? = ΔH1 − ΔH2
ΔH? = (−2326) − (−2220) = −106 kJ mol⁻¹
4. Constructing and Using Hess’s Cycles
- Identify known enthalpy values (formation or combustion).
- Draw an enthalpy cycle showing different pathways.
- Apply Hess’s Law equations to calculate the unknown enthalpy change.
Summary
- Hess’s Law simplifies thermodynamic calculations by breaking a reaction into steps.
- Apply known enthalpy values and combine them to find total ΔH.
- You don’t need to run the full reaction — just combine known reactions!
